Nitric Oxide in the Tumor Microenvironment of Triple Negative Breast Cancer
PROJECT CO-LEADERS:
Narendiran Rajasekaran, PhD, Assistant Professor, Northern Arizona University
William R. Montfort, PhD, Professor, University of Arizona
A. SPECIFIC AIMS. Enhancing immune clearance of tumor cells is an exciting advance in cancer therapeutics, yet many patients do not respond, and some endure serious side effects. A better understanding of mechanism in immune clearance is required for progress to be made. We seek to understand the role of nitric oxide (NO) signaling in the tumor microenvironment of triple negative breast cancer (TNBC), a common metastatic cancer with few treatment options. NO production in TNBC correlates with poor outcomes but the underlying reasons are unclear. We hypothesize that (a) NO directly inhibits prolyl hydroxylase, leading to stimulation of hypoxia inducible factor 1a (HIF-1a) and initiating a feed-forward cycle of tumor aggression; (b) loss of soluble guanylyl cyclase (sGC) activity removes a key checkpoint for tumor proliferation; and (c) that NO production leads to immune evasion in the tumor microenvironment as well as vascularization and recruitment of tumor suppressing macrophages. Our preliminary data suggest immune evasion is due in part to NO-dependent increased expression of CD47, which dampens both innate and adaptive immune responses. Our data also suggest NO-dependent release of cytokines by the tumor contributes by attracting tumor-supportive M2 macrophages and myeloid-derived suppressor cells (MDSC) while repelling natural killer cells and cytotoxic T lymphocytes.
Metastatic breast cancer, including TNBC, disproportionately effects Native American women, including Navajo women, who form the largest population of Native American women in Arizona. TNBC is more frequent in Native American women (~15% of all breast cancer cases) than in non-Hispanic White women (~12%). Our studies will target this unmet need in the Native American population and in the laboratory train Native American students for a career in basic translational research.
Here, we propose to (1) examine how NO signaling enhances tumor growth and invasion in TNBC; (2) uncover how NO signaling promotes immune evasion; and (3) enhance research capacity in cancer biology for Native American students in Arizona. These aims are part of a larger collaborative effort that includes clinical studies with University of Arizona Professor Pavani Chalasani (breast cancer oncology) and the Metastatic Breast Cancer focus group.
Aim 1: Uncovering How Nitric Oxide Promotes Tumor Progression in TNBC. We have discovered that NO promotes HIF-1a stabilization and a strong hypoxia gene expression profile that can drive tumor progression. We propose uncovering the underlying mechanism behind this observation, laying the groundwork for new therapeutic approaches. We will use human MCF10a immortalized normal breast cells, non-metastatic MCF7 cells and MDA-MB-468 TNBC cells for cell-based studies, and murine 4T1 cells for in vivo studies in immunocompetent mice. Our approaches will include monitoring of protein and message levels, RNA-seq, gene editing with CRISPR/Cas9, and immunohistochemistry of human and murine tumors. We will investigate sGC, the NO receptor, as a tumor suppressor. The activity of sGC stimulators will be examined, and inhibitors of NOS2, EGFR, HIF1a and PIM1 kinase.
Aim 2: Uncovering How Nitric Oxide Promotes Immune evasion in TNBC Our preliminary data suggest excess NO production in TNBC leads to production of cytokine and checkpoint blockade membrane proteins that promote tumor escape from immune clearance, particularly with respect to innate immunity. We will address how NO production in TNBC alters M1 macrophage and NK cell activation and migration, examining secretion of inhibitory cytokines and chemokines, and expression of inhibitory receptors, particularly CD47 and PD-L1. Both cell (MCF10a, MCF7 and MDA-MB-468) and mouse (4T1) models will be examined. These experiments are expected to reveal key factors limiting NK and M1 macrophage cell migration into the tumor microenvironment and tumor cell clearance, providing new targets for breast cancer therapy. We also expect to uncover NO-dependent recruitment of M2 macrophages, which are tumor promoting.
Aim 3: Increasing Research Opportunities and Capacity in Cancer Biology for Native American Students. We will increase the number of Native American students in cancer research at both the University of Arizona and NAU, and expand cancer research capacity at NAU, working closely with the Research Education and Outreach cores. Prof. Montfort and Assistant Prof. Rajasekaran have budgeted funding for a Native American trainee in each year of this proposal. Prof. Montfort will also work closely with early stage investigator Assistant Prof. Rajasekaran on grant and paper submissions and generally advise him on how to successfully establish the Rajasekaran laboratory.
RELEVANCE TO THE NACP
Our goals for participating in the Program for Native American Cancer Prevention are to (1) generate new anticancer targets for treating metastatic breast cancer in Native American women; (2) enhance research opportunities for Native American students; and (3) increase cancer research capacity at Northern Arizona University.
According to the American Indian Cancer Foundation “American Indians face alarming inequities in cancer incidence and mortality” (2). Cancer death rates increased in Native Americans in the 20 years 1990-2009 while they decreased in Whites. Breast cancer survival as measured by mortality-to-incidence ratios (MIRs) was worse among American Indian and Alaska Native (AI/AN) women compared to White women (3). Overall, Native American women have a 30-70% higher risk of dying from breast cancer when compared to other ethnicities (4,5). Importantly, triple-negative breast cancer (TNBC) incidence is higher in AI/AN women (14.6% of all breast cancer) then in white women (11.6%) (6). AI/AN women are more likely to be diagnosed with late-stage disease, in part due to lower access to care, and this leads to poor treatment outcomes and increased mortality. Whether there is also a contributing biological component specific to AI/AN women is unknown. Regardless, treatment strategies for metastatic breast cancer are limited and new therapeutic targets are greatly needed.
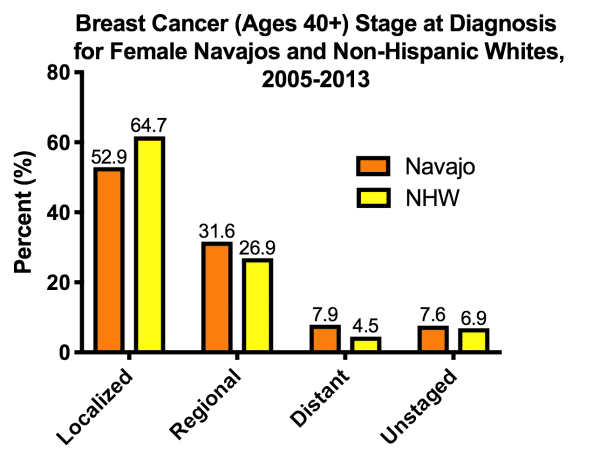
Breast cancer is the top incidence of cancer in Native American women of the Southwest, including women of the Navajo Nation (1,7). Breast cancer incidence is somewhat lower for Navajo women than for non-Hispanic White women. However, spread of the disease, including distant metastases, is worse (Fig. 1). These breast cancer disparities within the Native American population are compounded by resistance to existing therapies and a high relapse rate. Importantly, the NACP Community Advisory Committee (CAC) emphasize breast cancer as a high priority area of community interest.
University of Arizona. Research at the University of Arizona will be directed by Prof. William Montfort (Dept. of Chemistry and Biochemistry). Prof. Montfort has been a member of the University of Arizona Cancer Center (UACC) since 1990 and Co-lead for the UACC Therapeutic Development Program since 2015. He has been associated with the NACP for 10 years. Prof. Montfort has been continuously funded for 30 years and has trained over 80 students, of whom 20 are underrepresented and 6 who are Native American. Current Native American students Roslyn Curry (Navajo) and Shane Littlefoot (Navajo) are part of the proposed project. Previous Native American undergraduate Kyle Lopez is now a graduate student at UCSF; Native American graduate student Toni Green obtained a PhD and undertook Postdoctoral studies at the Roseman Cancer Research Institute; and post-baccalaureate student Anthony Ward became a research technician in the UA College of Nursing. Prof. Montfort has 28 publications with undergraduate and graduate student trainees. He has also been a member of >10 junior faculty mentoring committees and previously helped former NAU Assistant Professor Matthew Gage develop his laboratory and achieve tenure.
Northern Arizona University. Research at Northern Arizona University will be directed by Assistant Prof. Naren Rajasekaran, newly recruited to NAU through a joint effort involving the NACP. Assistant Prof. Rajasekaran is a UACC member and participating in the Therapeutic Development Program. His research in immunobiology is complementary to the biochemical focus of the Montfort laboratory. Assistant Prof. Rajasekaran’s primary home is in the Department of Chemistry and Biochemistry, where he is establishing a laboratory with extensive opportunities for Native American students in cancer biology.
Proposed Activities. In addition to cancer research, Prof. Montfort and Assistant Prof. Rajasekaran will actively recruit Native American students into their laboratories and guide them toward successful careers. We will work with the NACP Research Education Core and others on each campus to identify and recruit Native American students, and work with these students to ensure strong career progress, in concert with the Planning and Evaluation Core. Prof. Montfort will assist Prof. Rajasekaran in establishing his laboratory through advising on research directions (both in NACP and in his other U54 core project concerning reovirus), and providing feedback on manuscripts and grant application. Profs. Montfort and Rajasekaran will work with NACP Outreach Core and Community Action Committee (CAC) to connect with the Native American communities in Arizona, learn of their concerns and needs, and explore how we might work together to improve cancer outcomes. A particular focus will be on recruiting Native American students into research careers. We will also work with reservation healthcare providers to provide timely information on research leading to new treatment strategies.