Research
Biomaterial development
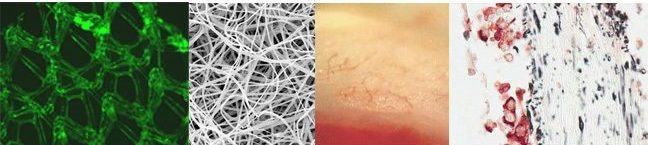
In the TERM lab, we develop, modify, and evaluate various biomaterials. In the above image are a few examples of our biomaterials efforts. Starting from the left image, a hernia patch (polypropylene mesh) is shown that has been surfaced modified with an extracellular matrix to help minimize inflammation. Next, an electrospun tropoelastin scaffold is illustrated, for use in the development of a wound healing device. To the right, a cardiac patch technology is shown, that has been implanted onto an acutely infarcted left ventricle. Notice the new small microvasculature that has developed within the patch after 2 weeks of implantation. Lastly, the far right image demonstrates a double-labeled immunohistochemistry sample from a co-cultured cardiac patch where the blue vimentin-reacted fibroblasts are co-localized with the red troponin-reacted cardiomyocytes.
Bioengineering replacement skin
(Left image) Native decellularized (without cells) skin with a 59% porous architecture.
(Right image) Bioengineered replacement skin, electrospun using skin proteins for wound healing applications with a 59% porous architecture. This research is being performed by NAU undergraduate Jordan Muller and Ph.D. graduate student Robert Diller in Dr. Robert Kellar’s TERM laboratory.
Biomaterial activation of platelet-rich plasma for wound healing applications
(From left to right): Far left is an SEM image depicting whole blood containing erythrocyte(s) and an inactive platelet. Middle image contains platelets activated with a collagen scaffold. Far right is a highly magnified image showing platelet vesicular release. These images were created by Aaron J Tabor, Ph.D., in the TERM laboratory of Dr. Robert Kellar.
Bench top (in vitro) wound healing assay
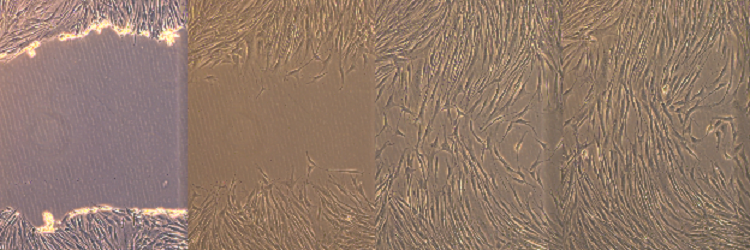
(100x) The above image demonstrates an in vitro “wound healing” scratch assay that was developed in the TERM laboratory by Bronson Pinto (Bioengineering Ph.D. Graduate Student). The scratch assay has been used in the lab as an early study tool to evaluate the effects of both soluble and insoluble compounds on wound closure (e.g. dermal fibroblast migration and proliferation). The assay allows for accurate, cost effective and high throughput experimentation. The starting wound width is shown in the far left image and closes over time (from left to right).
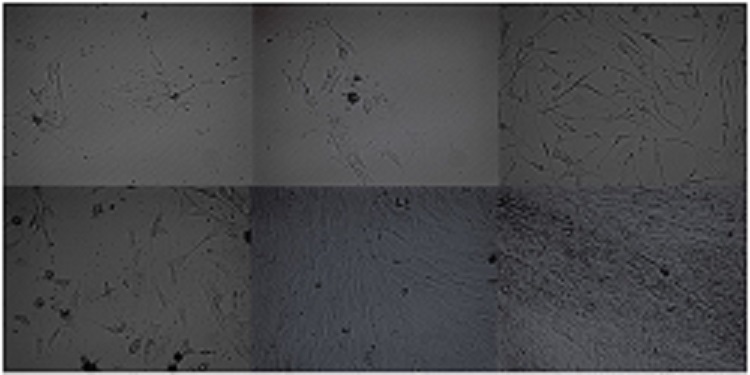
Culture of isolated white and brown adipose-derived stem cell
Stem cells were isolated from brown adipose tissue (BAT) and white adipose tissue (WAT), cultured, and examined at two day time points using an inverted microscope. The following images show the difference in growth between BAT and WAT derived stem cells.
(Top images, from left to right): Brown adipose-derived stem cells at day 4, 6, and 8.
(Bottom images, from left to right): White adipose-derived stem cells at day 4, 6, and 8. WAT stem cells reached confluence one week post-isolation, while BAT stem cells reached approximately 40% confluence. This research was performed by Martha Fowler (NAU Biology M.S. Student).